Certification
-
Cretification Process
Verification and Validation
Condation Of certification
BQSI is an accredited associated Certification Body for providing managementsystem certification services.
Certification services are provided based on the principles of Impartiality, Competence, Responsibility, Openness,confidentiality and responsive to complaints.All Management System Certification services are delivered within a frame of conditions applicable from initialreview of inquiry to issue of certificate of compliance and post certification activities as applicable.Compliance to conditions of certification shall be mandatory upon the client’s signing of certification agreement. BQSI reserves the right to make any changes in conditions of certification. However, such changes shall be
Communicated to client listed in certification directory.
1.0. DEFINITIONS
1.1. BQSI, British Quality System Institution (BQSI) is an independent third party certification body providing certification services of the Management System.
1.2. Client: An applicant organization applying to BQSI by providing client information for certification andsubsequent signing of certificate agreement for Management System Certification.
1.3. Certificate of compliance: Document issued to client after the satisfactory assessment of client’s ManagementSystem meeting the requirement of the contractual standard. The certificate of compliance demonstrates theeffective implementation of the Management System and confidence in the product and services provided as definedin the scope of certification. Clients have got the right to use the certification and accreditation body logo as per theinstructions provided along with certificate of compliance. Certificate of compliance is identified by certificatenumber specific to each client and is not transferable. Certificate of compliance is valid for three years from the dateof certification as specified in the Certificate.Certificate of compliance issued to a client is a demonstration of their capability to develop and effectivelyimplement a management system meeting the contractual requirements for a specific scope of product category.Certificate of compliance is not a product certification.
2.0. Confidentiality and Impartiality
All personnel associated with service delivery process of BQSI including members of various committees shall keepall information pertaining to business / process / activities obtained during the service delivery process asconfidential; Communication of confidential information to any other person or organization shall be with specificapproval of client and as per any local regulation, if applicable. If the local laws as legal requirements permitdisclosing of confidential information, BQSI may communicate such information for a specific purpose with priorpermission from the client.
BQSI maintains impartiality in all phases of certification service delivery process. Impartiality of services is ensuredthrough a committee to safeguard impartiality.
3.0.Certification Agreement
BQSI shall provide certification services to clients who have signed certification agreement and agreed to abide by Conditions for Certification provided in this document .Certification agreement is a legally enforceable agreement binding on both BQSI and the client. Certificationagreement shall be signed by client after review of proposal for certification and understanding the conditions for
Certification. The certification agreement is valid for an initial period of three years (3 years) and subsequentrenewals for subsequent three years (3 years) subject to satisfactory completion of periodic surveillance andrecertification audits as per the proposal for certification.In case non-compliance to any of the requirement(s) of certification agreement, BQSI reserve the right to initiate
actions for withdrawal, suspension, publication of transgression or other appropriate actions including legal action.However, BQSI shall provide .notification with adequate justification for initiating any such action(s).
4.0. Certification requirements:
Client organization shall:
4.1. Identify a management representative from its own management for:
4.1.1. Effective implementation of management systems and
4.1.2. Communications and coordination with BQSI.
4.2. Maintain a documented Management System in accordance with applicable contractual standard anddemonstrate effective implementation for a minimum period of three months prior to initial certification assessment.
4.3. Comply with all applicable legal requirements as any breach or contravention will be recognised asnonconformity during assessment and may have an impact on certification recommendation.
4.4. Conduct a minimum of one cycle of internal audit and one management review covering the specificmanagement system developed and implemented for certification prior to initial certification assessment.
4.5. BQSI nominated audit team members shall be provided access to all processes, production areas, personnel,applicable documents, records, organization structure, policy and procedures.
BQSI audit team may also comprise audit observers from BQSI, accreditation body representatives and other relevantauthorities as applicable. BQSI shall communicate to client on participation of BQSI audit observers and accreditationbody observers in the audit team. BQSI audit observers shall accompany any one of BQSI audit team members butshall not be auditing independently. Accreditation body audit observers shall observe the auditing process of BQSI but shall not be auditing the client management system independently.
4.6. Accreditation Body may conduct planned management system validation visits
4.7. Provide facilities needed by the audit team
4.8. Arrange guides to audit team with responsibilities to take the auditors to different functions, introduce theauditors to the auditee and resolve any communication issues during the audit. Guides will not participate in theaudit.
4.9.Ensure that consultants involved in the development of their management system do not participate in the audit.
5.0. Responsibilities and rights
5.1. Client
5.1.1. Review and understand the purpose of conditions for certification
5.1.2. Maintain records of certification documents issued by BQSI at all times and accessible to responsiblefunctions and interested parties as and when requested including accreditation body.
5.1.3. Respond to BQSI requests and correspondences within a reasonable time frame as requested
5.1.4. Cooperate with BQSI for conducting Initial Certification assessment, surveillance audits and recertification audits at agreed frequencies without undue delay.
5.1.5. Provide corrective actions for the identified non-conformances identified during Initial Certificationassessment, surveillance audits and recertification audits within 30 days of last day of the audit
5.1.6. Coordinate for conducting 1st surveillance audit within 12 months from the date of closing meeting of theinitial certification audit and subsequent surveillance audits as per agreed terms and conditions.
5.1.7. Coordinate for recertification audit at least one month prior to expiry of the certificate of compliance andpropose corrective action(s) for any non-conformance prior to the expiry of the certificate of compliance
5.1.8. Develop and effectively implement a documented systems for adequacy to the contractual standard at alltimes
5.1.9. Appoint a management representative to effectively implement and monitor the management systemsthrough internal audits and management review at planned frequencies.
5.1.10. Communicate with BQSI as and when required during the certification validity period.
5.1.11. Provide factual information on the organization structure, man power, statutory and legal requirements andcustomer complaints
5.1.12. Do not promote the certification status during suspension period of management system certification
5.1.13. In case of withdrawal of certificate due to any reason, client shall return back the original certificate to BQSIand discontinue use of logo on all advertising material.
5.1.14. Inform BQSI:
a. Any major organizational changes
b. Addition of new products and change in business processes with impact on the scope of certification
c. Changes relating to legal, commercial, ownership,
d. Major changes to organization structure and management personnel
e. Change in contact address and communication details
f. Addition or deletion in the number of branches, location and contacts which has impact on the scope ofcertification sites and size of the organisation
g. confidentiality of specific information, if required
h. Any ongoing legal issues pertaining to product, environmental or safety issues and their status including anyimpact on business activities.
i. Any disturbances with in the country with impact on audit schedule
5.1.15. Provide necessary working place, communication facilities and guides during assessments
5.1.16. Provide access to all records of customer complaints and corrective action taken as per the requirement ofimplemented management System
5.1.17. Comply with requirements of certification agreement including referred documents. Cooperate with BQSI incase of any legal actions initiated arising out of non-compliance with certification agreement.
5.1.18. Inform BQSI audit team on safety, emergency and security requirements to be observed within the plantareas
5.1.19. Multi site certification
5.1.19.1.All sites shall have a legal or contractual link with the identified central office of the organisation
5.1.19.2.All sites shall be implementing a common management system, which is laid down, established and subjectto continuous surveillance and internal audit by the identified central office.
5.1.19.3.Audit process will not be completed or delayed if any of the provisions for multi-site certifications are notmet.
5.1.19.4. In case of non-conformities are identified at central authority or any of audited sites during
certification process or during internal audit, requirement of client organization to review the non-conformities fortheir impact on overall system deficiencies as applicable to other sites.
a. If the non-conformities identified are analysed as detailed above, corrective action should be performed andverified at identified central authority and individual affected sites. If the analysis not done to evaluate the impact onoverall system deficiencies as applicable to other sites, justification for limiting the follow-up corrective action shallbe informed to BQSI.
b. BQSI reserve the right to increase the sample size to establish confidence in the certification.
c. Exclusion of any site during the audit or after the audit to overcome any findings of audit is not acceptable.Any required exclusion can be agreed prior to the audit
5.1.19.5. Understand that BQSI will not issue certificate of compliance in case of non-conformance issued at any siteunless resolved as detailed in 5.1.15.4
5.1.19.6. Understand that sites with non-conformances will not be allowed to be withdrawn from the scope of certification after completion of initial assessment
5.1.19.7. Cooperate with BQSI to conduct assessment of additional sites irrespective of the proposal for certification to gain confidence in the implementation across all the sites, in case of non-conformances issued at any of the sites and subsequent corrective action as detailed in 5.1.11.2.
5.1.19.8. Cooperate with BQSI to increase the frequency of samples or increase sample size to re-establish satisfactorycontrols by BQSI and at additional cost
5.1.19.9. Inform BQSI of closure of any branches covered by scope of certification. Failure of client to communicatesuch information to BQSI shall be considered as misuse of certification
5.1.19.10. Management process like Internal Audit, Management Review and data analysis shall cover all the siteswhich are identified for multi site Certification
5.1.20. Provide information on health, safety and environmental requirements to be followed by audit team duringthe assessment.
5.1.21. Inform BQSI audit team on confidential nature of any document
5.1.22. Provide information on the applicable statutory and regulatory requirements for the scope of certification.
5.1.23. Comply with certification requirements as communicated by BQSI including changes, statutory andregulatory requirements at all times.
5.1.24. Cooperate for short notice audits for:
a. Investigation of any complaint received from the interested parties,
b. Response to any changes
c. Follow up on suspended certificate of compliance
Agree for any additional visit at cost, which may be necessitated, from such short notice audits for investigation,which is outside the scope of proposal for certification.
5.1.25. Agree for follow-up audit, if recommended by Lead Auditor during any of the assessment and / orrequirement of the BQSI Certification Decision Committee.
5.1.26. Ensure proper use of certificate of compliance, quality mark, and accreditation mark when making referencein communication media as per the BQSI instructions
5.1.27. Inform BQSI for any change of audit team members/technical experts in advance with cause like conflict ofinterest.
5.1.28. Coordinate for planning and conducting surveillance audit and recertification audits at agreed frequency asper the certification agreement to ensure continued validity of the certificate of compliance.
5.1.29. Provide corrective action(s) for the non-compliances recorded during any assessments
5.1.30. Ensure use certificate of compliance in a planned and controlled manner which will not bring BQSI and Accreditation body to disrepute and loose trust of interested parties.
5.1.31. Change relevant documents within an agreed timeframe, in case of changes to conditions for certification, asand when communicated by BQSI.
5.1.32. Understand the certification process as detailed in 6.0 of conditions for certification
5.1.33. Maintain confidentiality of proceedings of the assessments
5.2. Integrated Quality Certification
5.2.1. Provide impartial certification services to clients with in the configuration of the accredited scope
5.2.2. Maintain confidentiality of the information obtained during the certification service delivery
5.2.3. Communicate requirement for certification to the client
5.2.4. Description of initial certification including the application, certificate maintenance and process for granting,maintaining, reducing, extending, suspending, withdrawing and recertification.
5.2.5. Communicate the professional service charges for certification services for 3 years period
5.2.6. Certification of multi site clients shall be done as per the requirements of IAF Mandatory Document IAF MD1.
5.2.7. Information on procedure for handling complaints and appeals
5.2.8. Communicate changes to certification criteria to clients for implementation with in an agreed time frameconsidering the views of interested parties
5.2.9. Provide adequate notice for surveillance and recertification audits
5.2.10. Provide all opportunities to client to explain their stand point for any identified non-compliance
5.2.11. Responsible and retain authority for decisions relating to certification, including granting, maintaining,renewing, extending scope, reducing scope, suspending and withdrawing of certification.
5.2.12. Responsive to complaints from interested parties
5.2.13. Ensure availability of publicly accessible information pertaining to certification process, list of certifiedclients and their status of certification
5.2.14. Multi site clients
5.2.14.1. Communicate the criteria for multi site application and Decision on eligibility of a client with multiple sitesfor sample audit as per IAF and accreditation body guidelines
5.2.14.2. Select appropriate sample size to gain confidence on the implementation of centrally controlled managementsystems in all branches prior to decision on certification
5.2.14.3. Provide an opportunity to client to withdraw branches which are not ready for certification from scope ofcertification prior to initial assessment
5.2.14.4. Increase the frequency of samples or increase sample size to re-establish satisfactory controls across the sites
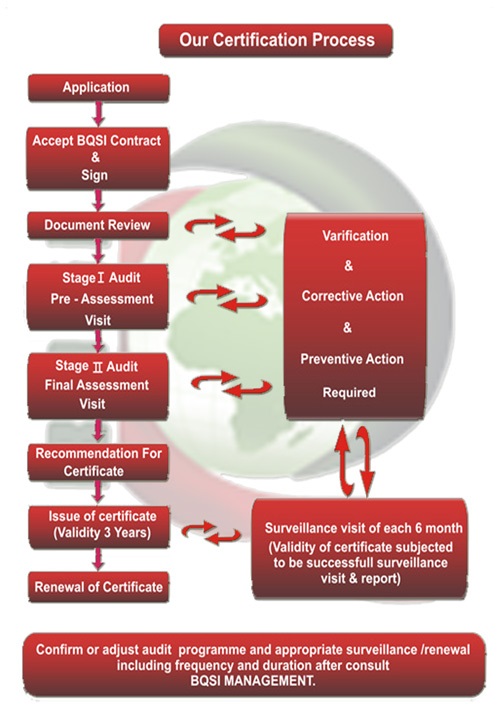
6.0. Certification process
6.1. Application
Information about the applicant organisation is gathered through client information for certification (CIC). Details such as scope of certification, man power, statutory & regulatory requirements, exclusions, processes and productinformation are critical inputs for certification process
6.2. Application Review
The submitted CIC is reviewed to ensure the adequacy of the information for submitting the proposal and subsequentprovision of certification services. The ability and competence to perform the certification is decided by BQSIconsidering its accreditation scope. BQSI shall submit the proposal for certification services for initial assessment andsurveillance audits for the 3 years period along with certification agreement and conditions for certification.
6.3. Proposal & Agreement
Commercial proposal is submitted to client providing information on number of audit Mandays required for each stageof audit process and professional charges associated. Upon acceptance of terms and condition stated in the proposaland client acceptance to condition for certification, a certification agreement is signed between BQSI& Client.Agreement is signed in two originals. One is retained with client and other with BQSI.Subject to approval by client, BQSI may offer services for an unaccredited certificate. BQSI may plan to transfer suchcertificate to accredited certificate subject to approval by accreditation body with in a time frame.
6.4. Initial Certification Audit
Initial Certification Audit is conducted to evaluate the implemented management system and
Decide on the maturity of the system and issue certificate of compliance. Initial assessment is conductedin two stages as per the requirement of ISO 17021 & ISO 22003 Audit team leader and audit team of BQSI are responsible to for the audit and control of the audit execution as peraudit plan
- Stage I Audit: Conducted to assess the management system planning, validate the information provided in theclient information for certification, required logistics and planning for Stage II assessment
- Stage II Audit: Verify compliance of the management systems to the planned arrangements and decide on therecommendation for certification based on assessment output.
6.4.1.Upon signing of the certification agreement, client shall coordinate the date for stage I audit. BQSI shall informclient of the stage I audit schedule. Stage 1 is conducted to evaluate site specific conditions, focus on ManagementSystem planning and planning for stage II audit, document review to evaluate the adequacy of the document to theapplicable standard, allocation of resources for stage II audit, understand the scope/clause exclusions/ applicablestatutory requirements/product standards/processes/evaluate client’s understanding of the applicable standard/aspect impactstudy for EMS and /hazard-risk analysis for OHSAS and HACCP study for FSMSAssessment report shall be provided to client along with audit observations, if any. Client is responsible to plan foradequate corrective actions for audit observations along with revision to system documents, if required. Client shallcommunicate the corrective actions to BQSI prior to stage II assessment. Output of stage I assessment and nature ofobservations may have impact on the stage II assessment schedule. Inadequate and ineffective corrective actions forstage I assessment may lead to major non-conformances in stage II assessment.
6.4.2.Stage II audit
Stage II audit is conducted to evaluate effective implementation of the Management System.
6.4.2.1. Inform audit team nomination and audit programme in advance.
6.4.2.2. Conduct opening meeting to explain audit methodology
6.4.2.3. Verify effective implementation of the management System for adequacy to the scope of certification byexamining personnel, policies, procedure and records on sample basis against the contractual standard.
6.4.2.4. Stage II audit is carried our as per the checklist. Check list is an internal document of BQSI and is used toassist auditors during the audit.
6.4.2.5. Record Non conformance and classify as Major or Minor
6.4.2.6. Conduct closing meeting to explain audit findings, recommendations and revision to scope of certificationsif required.
6.4.2.7. Provide copy of assessment report along with non-conformance report if any and recommendationsincluding schedule for surveillance / recertification audit.
6.4.2.8. Review the suitability of surveillance frequency and/or man days based on the audit findings.
6.4.2.9. Review corrective actions provided by client for all the non-conformance reports by follow up audit ordocumentation verification.
6.4.2.10. Audit team may terminate the audit prematurely if there is no evidence of implementation of plannedmanagement system and / or compliance to applicable legal requirements
6.5. Assessment Report Review and Issue of Certificate of Compliance
6.5.1. Assessment Review of audit reports by BQSI certification decision committee. Impartiality shall be maintainedduring such reviews.
6.5.2. Resolutions of clarifications in the audit reports if any prior to approval of recommendations.
6.5.3. Issue certificate of compliance within 10 days after approval by certification decision committee, which is validfor 3 years from the date of certification decision. BQSI maintains list of certified clients in web sitewww.BQSIglobal.com and also accreditation body web site, if applicable. clients shall verify the BQSI web site for thereference of their organization in the web site and contact BQSI Corporate office for any clarification.
6.5.4. Certificate of compliance is considered invalid under following conditions.
a. Client organization is not listed in BQSI web site and / or in Accreditation Body web site, if applicable
b. Certificate of compliance is not having certificate numberClient shall verify the validity of the issued certificate with BQSI Corporate office with following detailsClient shall not accept Certificate of compliance from any other sources except from BQSI Corporate office unlessotherwise informed of alternative arrangement for delivery of certificate of compliance.
6.5.5. Issue instructions on the use of quality marks and accreditation mark
6.6. Surveillance Audit
6.6.1.Conduct surveillance audit at agreed frequency to verify continued implementation of Management System asper the proposal. First surveillance audit shall be conducted within 12 months from the closing meeting ofthe initial certification stage II audit.
6.6.2. Verify the effectiveness of continuous implementation of Management System and planned processes duringeach surveillance audit ensuring to cover all processes at least once during each certification cycle.
6.6.3. Conduct follow-up audit, if required.
6.6.4. Verify use of Quality mark and accreditation mark as per BQSI instructions.
6.6.5. Provide recommendations on continuation of certification as per audit report provided at the conclusion of eachsurveillance audit.
6.7. Re-certification
6.7.1. Conduct re-certification audit prior to certification period for continuation of certificate of compliance andsubsequently followed up by Surveillance audits as per the accepted proposal. Re-certification audit shall becompleted before the expiry of validity of the previously issued certificate of compliance
6.8. Scope Extension / Scope Reduction
6.8. 1.Scope Extension
Scope extension audits shall be conducted upon request from client organization under following conditions.
6.8.1.1. Inclusion of process (es) in the system which was earlier excluded. Example: Design and Development
6.8.1.2. Inclusion of additional product(s)
Request for scope extension shall be reviewed and conducted as detailed in clause 6.1 to 6.5 except that requirementfor conducting stage 1 audit may be waived with justification. Scope extension audit may be conducted as a separateassessment or combined with surveillance or recertification audit.
6.8.2. Scope Reduction
Scope of certification may be considered for scope reduction under following conditions
6.8.2.1. Client discontinuation of product from manufacturing as and when requested by client
6.8.2.2. Restriction on sale of certain product(s) by regulatory authorities
6.8.2.3. Inadequate corrective action for complaints by regulatory authorities
6.9 Short Notice Audit
Condition arising out of a complaint from interested parties including regulatory authorities under which audit has tobe planned preliminarily for the purpose of investigation and review planned corrective action. Upon receipt ofcomplaint, BQSI shall communicate with the client immediately on the requirement for an immediate audit andcoordinate for an audit schedule.Audit shall be planned preferably after completion of initial investigation by BQSI client. Time frame for planning theaudit shall be appropriate to the nature of complaint. If the complaint has an impact on the interested parties health andsafety, audit shall be planned within a maximum of 7 working days.
6.10 Transfer Audit
Recognition of an existing and valid management system certification, granted by one accredited certification body(Issuing certification body), by another accredited certification body (accepting certification body) for the purpose ofissuing its own certificate. Following documents shall be submitted by client for review.
a. Copy of certificate of compliance from the previous certification body. Review the certificate of compliance for thescope, EA / NACE classification and the capability of BQSI to provide accredited certificate.
b. Copy of assessment report of previous certification body. Review for any adverse remarks in the report.
c. Copy of NCRs of previous certification body. Verify if the NCRs are major or minor and open or closed. Reviewthe criticality of the report.
d. Compliance status to applicable legal requirements (EMS , OHSAS and FSMS)
e. List of aspects and impacts identified (EMS)
f. List of Hazards identified (OHSAS)
g. List of HACCP Studies (FSMS)
h. Copy of last internal audit report and management review. Review for meeting the requirement of the contractualstandard.
6.11 Criteria for Suspension and withdrawal of Certificate
6.11.1 Certificate may be withdrawn under following conditions.
6.11.1.1 At the request of the client
6.11.1.2 Client does not comply with any part of the conditions of certification.
6.11.1.3
a) Client fails to offer surveillance audit as per the agreed frequency. Certificate may be kept undersuspension for a maximum period of six months from the due date of surveillance audit.
6.11.1.4 Recertification audits are not offered by the client prior to the expiry of the validity period of thecertificateBQSI adopts a policy of providing continuous services based on regular surveillance to client without any lapse on theprocedural requirements to maintain the certification. BQSI forwards the surveillance audit notifications to clientorganization as a reminder of the forthcoming /delayed surveillance audit to ensure that validity of the certificate ofcompliance is maintained. BQSI provides an opportunity to client to initiate action but without any responsibility forcoordinating the surveillance audit.Following is the plan of action of BQSI to enable client to coordinate for the surveillance audit within the agreed timeframe.a. Surveillance Notification letter 1 is forwarded to client two months ahead of the due date of surveillanceaudit.
b). Surveillance Notification letter 2 is forwarded to client one month ahead of the due date of surveillance audit.
c). Surveillance Notification letter 3 is forwarded to client on the due date of surveillance audit, informing theclient of keeping the Certificate of Compliance under suspension for a period of 6 months from the due date andprovide an opportunity to the client to coordinate for the surveillance audit. If any surveillance audit is to be conductedat this period, a complete audit shall be planned to cover all the processes of Management System. Any additionalcharges towards increased man days shall be charged to the client.
d) Withdrawal notification is forwarded to client Six months after the expiry of the due date of surveillanceaudit informing the client regarding the withdrawal of certificate of compliance with immediate effect and copymarked to accreditation body, Quality Council of India and the relevant ministry, in case of small scale industry.
e). If the client wishes to re-apply for certification after withdrawal same will be considered as a new contract.
Certificate of Compliance shall be withdrawn or scope of certification of client’s management system shall bereduced (for client failed to meet the requirements for those part of the scope), when the client does not complywith any part of the conditions of Certification. When the certificate of compliance is suspended/ withdrawn,certified organization directory including information on BQSI web site is updated accordingly.
Client shall return the certificate of compliance to BQSI corporate office immediately and discontinue use of reference
to certificate of compliance.
6.12 Inactive certification
Certified management system may be kept as inactive when:
6.12.1 Client makes a request in view of current market situation. The current certification shall be allowed tocontinue for a period up to next surveillance due.
6.12.2 Request for further extension may be reviewed and granted by certification Decision committee. Suchextension shall not be more than two surveillance duration from the certification time. A fresh audit shall be conductedbefore revoking the inactive certificate.
6.12.3 BQSI shall withdraw the certificate of compliance after the above period, with advance notice to client andrequesting to return the original certificate.
6.13 Use of quality mark and accreditation mark
6.13.1 Accreditation mark shall be used only in conjunction with BQSI Quality Mark.
6.13.2 The size of the accreditation shall be the same as the quality mark.
6.13.3 The Quality mark and accreditation mark may only be used on correspondence, advertising, invoice,stock form and promotional material for the products or services described in the scope of certification. Accreditationmark shall not be used on business cards.
6.13.4 On size A4 stationery the Quality mark and accreditation mark shall be 15 x12 mm.
6.13.5 Proportional increase/reductions may be allowed on stationary of larger/smaller size than A4 and shall belegible to have Management System standard and certificate number without any distortion or overlapping.
6.13.6 The conditions as indicated shall also apply to packaging material and promotional products. The partyreporting the violations to the attention of BQSI is informed of appropriate action being taken but is not provided with
the details as it may be violating right to confidentiality.
6.13.7 The certification mark shall be produced in single colour either deep blue or red. Use of other colourwill require specific approval of BQSI.
6.13.8 Any deviation or specific use of mark for special purposes like small advertisements, on client vehiclesshall not be allowed.
6.13.9The mark shall not be displayed on vehicles except in publicity material like part of a large advertisement.
6.13.10 The Accreditation mark shall only be printed in the colour combination or in the grey-black
combination as specified in the instructions attached to certificate of compliance.
6.13.11 The mark shall not be used on any inspection reports, calibration certificates, laboratory test
certificatesetc as such reports are deemed to be products for such organisations
6.13.12 Mark shall not be used on business cards
6.13.13 The certified organization shall abide by the BQSI rules of certification to discontinue any use ofQuality mark and Accreditation mark that is unacceptable to BQSI.
6.13.14 BQSI shall initiate corrective action with the certified organization to avoid misuse of the Quality markand Accreditation mark brought to the notice of BQSI by any interested parties and/or general public subject tothorough investigation.
6.13.15 Upon withdrawal of certification by BQSI or upon request by client to withdraw certification or due tocancellation of certification contract with BQSI, the certified organization shall immediately discontinue use of allmarks and to destroy all stocks of material on which they appear.
6.13.16 Usage of marks shall be verified during each subsequent audit and recertification audit and findingsreported in assessment report. Misuse of quality marks, if any, shall be recorded as non-conformance and correctiveaction taken verified prior to continuation of certification or reissue of certificate. Misuse may also be reported by anyinterested party and BQSI shall take action against misuse.
7 .Complaints, Disputes and Appeals
7.11 BQSI has established an impartial appeals committee constituted by Executive Director to investigatecomplaints, appeals and disputes related to the certification services
7.12 Analyse and take corrective actions and inform client on the action taken
7.13 Provide an opportunity to the client to appeal against actions taken by BQSI appeals committee
7.14 Appeals committee shall prepare a report after investigation including providing an opportunity to theclient to represent their evidence.
7.15 Decision of the Executive Director who is responsible for the approval of report is binding on both theparties
8 .Certification Service Professional Charges
8.11 BQSI shall submit the invoices for the professional services as indicated in proposal for certification, for theclient approval and payment within 15 days.
8.12 Certificate of compliance shall be forwarded after receipt of the professional service charges as per proposal.
9 .Liability of BQSI
Certification services are provided by BQSI as per the agreed proposal for certification Liability of BQSI shall belimited to the commercial terms referred in the proposal under any circumstances. Client agrees to indemnify, holdharmless and defend BQSI from any and all liability of any and all kinds and types, including without limitation,claims, demands, or causes of action, including attorney fees, made or brought by any entity, person, firm orcorporation arising out of or incidental to the certification services to be provided in connection with this agreement byreason of injury of any entity, person or damage of any property regardless of whether such injury or loss, cost,damage or expenses is occasioned in whole or partly by any negligent or omission on the part of BQSI, its
Subcontractors or employees and regardless of where any such loss or any action may occur.Client agree to indemnify and hold BQSI harmless from and against any fines, taxes, levies imposed which may beasserted or imposed upon BQSI by any country. Client shall also indemnify and hold and save BQSI against all expensesand out-of-pocket expenses incurred by BQSI in connection with or related to the assertion by any such country orjurisdiction of liability of BQSI to pay any such fine, tax, and levy imposed.This Agreement shall be governed by and construed and enforced in accordance with the laws and subject tojurisdiction of courts at UK.
10. Validity and authenticity of the certificate of compliance
BQSI updates the webpage www.bqsi.co.uk of all certified clients. Client is requested to report any discrepancy in issued certificate of compliance to BQSI Corporate Office and write us at contact@bqsi.co.uk.


 2013 BRITISH QUALITY SYSTEM INSTITUTION | ALL RIGHT RESERVED
2013 BRITISH QUALITY SYSTEM INSTITUTION | ALL RIGHT RESERVED 